The first adolescent case infected with chikungunya virus in South Korea
Article information
Abstract
Chikungunya fever, a viral illness transmitted to humans through the bites of infected mosquitoes, presents with symptoms such as high fever, severe myalgia, headache, arthralgia, rash, and vomiting. This disease predominantly manifests in Southeast Asia, Africa, and Central and South America, with a limited occurrence in Northeast Asia. To date, no such a documented case has been reported in South Korea. Herein, we present the first adolescent case of chikungunya fever in South Korea following a travel to Bali, Indonesia.
Introduction
Chikungunya fever is an infectious disease caused by chikungunya virus transmitted by mosquitoes, mostly Aedes aegypti or Aedes albopictus. The incubation period is generally 1-12 days; generally, it takes 3-7 days for the symptoms of this disease to appear (1). The symptoms include a sudden-onset fever during the acute phase, which lasts for 7-10 days. In addition, arthralgia, rashes, headache, fatigue, nausea, vomiting, myalgia, and polyarthritis may occur during the disease course. Approximately 3%-28% of infected individuals may not show symptoms (1), and in some patients, it may cause neurological complications, such as meningitis, Guillain-Barré syndrome, central nervous system lesions, myocarditis, and hepatitis. Joint-related symptoms can last from 3 weeks to 3 months after the acute phase (1,2).
Chikungunya fever was first reported in Tanzania in 1952 as an acute infectious disease characterized by fever, rash, and arthralgia. Since then, outbreaks have continued sporadically in Africa and Asia (3-5). Since 2005, more than 1,900,000 patients have been reported in Southeast Asian countries. Notably, in the summer of 2007, an epidemic of chikungunya fever occurred for the first time in Europe, rapidly spreading to visitors to India from Italy, resulting in approximately 200 patients (5). South Korean government designated chikungunya fever as a group 3 legal infectious disease, and the first domestic case was reported on July 29, 2013 (1,3,5). However, to the authors’ knowledge, no such a pediatric case has been reported in the country (Fig. 1) (6).
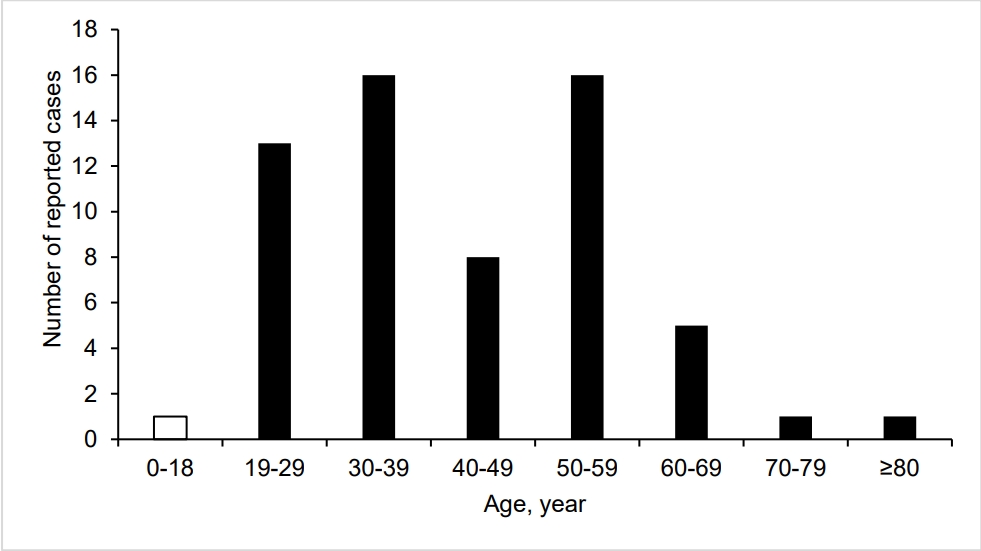
Age group distribution of cases of chikungunya fever in South Korea, from January 2000 through February 2024. This current case belongs to the age group of 0-18 years (open bar).
Here, we report the first case of chikungunya fever in an adolescent boy who visited the hospital with symptoms of fever and rash after traveling to Bali, Indonesia. This case report was reviewed by the research review committee of Severance Hospital, Yonsei University College of Medicine, and the requirement for written consent was waived (IRB no. 4-2024-0014).
Case report
A previously healthy, 14-year-old boy visited the pediatric emergency department (ED) with fever and truncal rash, which had started 3 and 2 days before the visit, respectively. He entered South Korea 3 days before the visit, after traveling to Bali. The rash spontaneously disappeared 3 hours after the onset. At the time of the visit, he reported 1 episode of vomiting, without residual skin lesions or respiratory symptoms.
The initial vital signs were as follows: blood pressure value, 129/80 mmHg; heart rate, 88 beats/minute; respiratory rate, 20 breaths/minute; temperature, 38.2 °C; and alert with acute ill appearance. The boy’s weight and height were 102.3 kg (99.7th percentile) and 177.5 cm (95.7th percentile), respectively, making a body mass index of 32.5 kg/m2 (> 99th percentile). Redness and edema were observed in the pharynx, and there were no abnormal findings in the heart or lungs. Despite the absence of abdominal tenderness, the liver and spleen were palpated 2 fingerbreadths below the right costal margin. He reported that 2 days before the visit, a mild papular rash was observed on the torso, back, and legs. The initial blood tests showed a mild thrombocytopenia, and increased concentrations of aminotransferases and C-reactive protein, with otherwise normal laboratory findings (Fig. 2). Normal findings were found on the initial chest radiograph. On the same day, he was hospitalized with a presumptive diagnosis of viral infection or a possible foreign infectious disease.
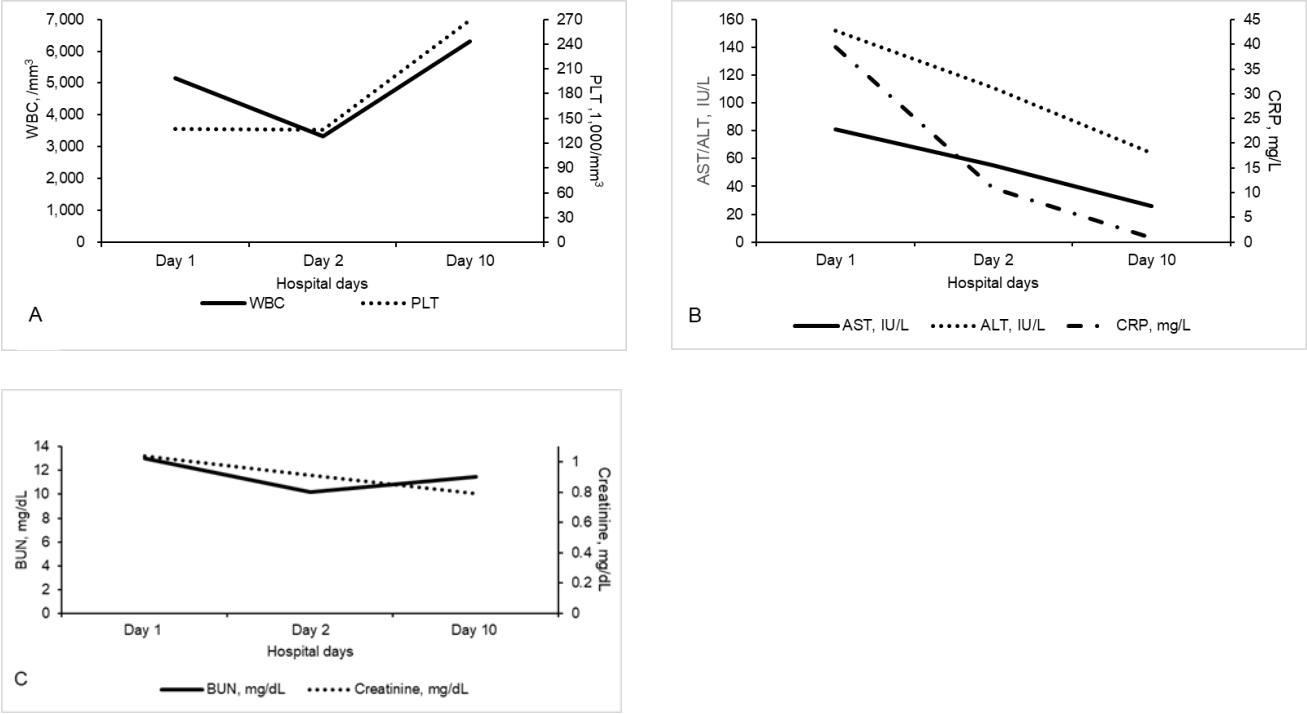
The changes in the 14-year-old boy’s values regarding WBCs, platelets (A), AST/ALT, CRP (B), and BUN/creatinine (C). On day 1, WBCs were 5,150/mm3 (neutrophils, 75.8%; lymphocytes, 9.4%; and monocytes, 10.7%); platelets, 137,000/mm3; AST/ALT, 81/152 IU/L; CRP, 39.4 mg/L (reference value, 0-8 mg/L); procalcitonin, 0.39 ng/mL (not shown [reference value, 0-0.5 ng/mL]); and BUN/creatinine, 13.0/1.04 mg/dL. On day 2, WBCs were 3,340/mm3 (neutrophils, 53.3%; lymphocytes, 32.0%; and monocytes, 13.5%); platelets, 136,000/mm3; AST/ALT, 55/111 IU/L; CRP, 11.0 mg/L; and BUN/creatinine, 10.2/0.91 mg/dL. On day 10, WBCs were 6,310/mm3 (neutrophils, 54.0%; lymphocytes, 31.9%; and monocytes, 11.1%); platelets, 269,000/mm3; AST/ALT, 26/64 IU/L; CRP, 0.9 mg/L; and BUN/creatinine, 11.5/0.79 mg/dL. WBC: white blood cell, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, BUN: blood urea nitrogen.
In the afternoon of day 1, negative findings were confirmed on the peripheral blood smear and rapid antigen tests for malaria. Subsequently, we found negative findings on the respiratory virus polymerase chain reaction panel (film array, BioFire), which identified 23 types of the viruses, such as severe acute respiratory syndrome coronavirus 2. On day 3, abdominal ultrasonography showed no specific findings other than mild hepatomegaly or fatty liver disease. The blood and urine cultures performed on day 1 showed no growth of microorganisms for 48 hours.
Considering that the boy had been to Indonesia, the Seoul Research Institute of Public Health and Environment was contacted to confirm whether he had dengue fever, Zika virus infection, or chikungunya fever. Blood samples and requests for testing for the 3 viruses were sent on day 2.
He received supportive therapy during hospitalization. Subsequently, the fever began to improve, and a follow-up peripheral blood test on day 2 showed mild leukopenia and decreases in concentrations of aminotransferases (Fig. 2). On that day, he had no further symptoms of fever or rash, and was in good general condition. On day 3, he was discharged uneventfully.
One week after discharge, i.e., day 10, the boy’s condition was monitored at the outpatient clinic. He was well-looking, had no complaints of fever, rash, or arthralgia, and showed improvement in the blood tests performed at the outpatient visit (Fig. 2). At that time, he was diagnosed with chikungunya fever based on the results of the tests that were requested to the abovementioned municipal institute. In detail, the results were positive findings of immunoglobulin M on an enzyme-linked immunosorbent assay for the chikungunya virus antibody, and of the chikungunya virus gene on a real-time polymerase chain reaction.
Discussion
Recently, the areas of outbreak of chikungunya fever are spreading rapidly worldwide due to the expansion of the habitat of the Aedes spp. mosquitoes caused by climate change, mutations in the chikungunya virus, and an increase in the number of overseas travelers (5,7). In South Korea, there has been no chikungunya epidemic, rendering the country an area with little immunity. Therefore, it is important to report and study the relevant cases because it can lead to a stronger spread. This current case is vital in that it is the first report of chikungunya fever infection in children or adolescents in South Korea. In light of the resurgence of overseas travel following the end of the coronavirus disease 2019 pandemic, our case underscores the importance of considering rare mosquito-borne diseases, such as chikungunya fever, in the differential diagnosis of post-travel fever presenting after international visits.
The case patient developed fever and rash after traveling to Bali, and showed a series of nonspecific laboratory findings of neutropenia, thrombocytopenia, increased concentrations of aminotransferases and C-reactive protein. Because he did not accurately remember being bitten by a mosquito or insect during the trip, a mosquito-borne infectious disease was not suspected at first. However, based on the travel history, fever, and rash, we requested tests for dengue fever, chikungunya fever, and Zika virus infection to the municipal institute. Despite being 3 separate infectious disease entities, they have similar manifestations and primary outbreak areas or climates, and are commonly transmitted by the Aedes mosquito. Children with chikungunya fever feature less prominent signs and less frequent hospitalization (8). This concept is in accordance with that in the current case, the transient rash could not be confirmed at the time of visit to the ED. Also, arthralgia, a characteristic symptom of chikungunya fever, was not observed. Dengue fever, chikungunya fever, and Zika virus infections are mostly self-limited. Our case shows the importance of identifying chikungunya fever even in the absence of arthralgia, a typical symptom of the disease entity.
Differential diagnosis should be performed when emergency physicians or pediatricians encounter children or adolescents presenting with fever and rash at EDs. However, as in the current case, chikungunya fever should be considered when adolescents with a history of overseas travel to high-risk areas present with fever, rash, leukopenia, thrombocytopenia, or increased concentrations of aminotransferases.
Notes
Author contributions
Conceptualization, Supervision, and Project administration: Ahn JG
Methodology and Investigation: Hwang EJ and Ahn JG
Validation and Formal analysis: all authors
Data curation and Visualization: Hwang EJ
Writing-original draft: Hwang EJ
Writing-review and editing: all authors
All authors read and approved the final manuscript.
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Funding sources
No funding source relevant to this article was reported.
Data availability
All data presented in this manuscript is available from the corresponding author upon reasonable request.
