An adolescent female with intentional ingestion of a large amount of metformin requiring extracorporeal membrane oxygenation and continuous renal replacement therapy
Article information
Abstract
Acute overdose of metformin can show potentially fatal lactic acidosis. Management should be directed towards close monitoring of renal function and hemodynamics. Patients may require dialysis or extracorporeal membrane oxygenation in cases of hemodynamic instability. This case presents an adolescent with massive metformin ingestion estimated at 100 g leading to metformin-associated lactic acidosis and subsequent respiratory failure, cardiovascular collapse, and acute kidney injury. The complications were successfully managed with venoarterial extracorporeal membrane oxygenation, continuous renal replacement therapy, and mechanical ventilation.
Introduction
Metformin is a biguanide commonly used to treat type 2 diabetes mellitus (1,2). While generally considered safe, metformin carries a black box warning for potentially fatal lactic acidosis seen both in therapeutic use and acute overdose (1). Reported cases have a 30%-50% mortality (3). Metformin suppresses hepatic gluconeogenesis, inhibits glycogen breakdown, and helps prevent insulin resistance in the liver and adipose tissue (1,4-6). It inhibits pyruvate carboxylase, which impairs the conversion of lactate to pyruvate, leading to impaired cellular respiration and eventually, lactate accumulation (3,7). Metformin-associated lactic acidosis (MALA) occurs when lactate concentration exceeds 5 mmol/L and pH is lower than 7.35, in association with metformin exposure (2). MALA is largely a type B lactic acidosis but can be confounded by type A in overdose (1,3,4). Renal impairment, hepatic dysfunction, and other processes that increase lactate production, such as sepsis and congestive heart failure, can further contribute to the likelihood of developing MALA (3). The use of extracorporeal removal techniques (ERTs), including dialysis and extracorporeal membrane oxygenation (ECMO) in drug overdose, has increased over time, from 6 episodes in 2000 to 88 episodes in 2018 (8).
We present the successful management of an adolescent female with intentional ingestion of 100 g of metformin who presented with severe lactic acidosis and rapid decompensation to respiratory failure, severe acute kidney injury, and ultimately cardiovascular collapse. This case report was approved by the institutional review board at Children’s Hospital of the King’s Daughters with a waiver for informed consent (IRB no. 23-06-NH-0187).
Case
A 13-year-old previously healthy girl, weighing 66 kg, presented to an outside emergency department after intentionally ingesting approximately 200 tablets of 500-mg metformin after a fight with her sister. This was an impulsive act. The girl had no prior psychiatric history, and later denied her suicidal intent. Initially, she had been alert with stable vital signs, noted to have emesis and urinary incontinence. The initial concentration of lactate was 5 mmol/L (reference value, 0.7-2.1 mmol/L). She was then transferred to our pediatric emergency department for a higher level of care.
En route, intravenous 5% dextrose and sodium bicarbonate were started. Shortly after arrival, she rapidly decompensated with acutely altered mental status, snoring respirations, and hemodynamic instability. The initial vital signs were as follows: blood pressure, 65/28 mmHg; heart rate, 99 beats/minute; respiratory rate, 18 breaths/minute; temperature, 33.8 。C; and oxygen saturation, 58% in room air. A point-of-care glucose value was < 20 mg/dL. Initial venous blood gas analysis showed a pH of 6.989, PaCO2 of 43.5 mmHg, PaO2 of 81 mmHg, HCO3 of 10.5 mmol/L, and base deficit of 21 mmol/L with a lactate concentration increased to 13.9 mmol/L. Three hours after the arrival, lactate increased to > 20 mmol/L. Results were negative for viral polymerase chain reaction, urine drug screen, and blood concentrations of alcohol, acetaminophen, and salicylate. Complete blood count and metabolic panel were unremarkable.
Due to her rapid decline, she was intubated and thus, did not undergo activated charcoal therapy and gastric lavage. She received 2 boluses of 0.9% saline, 50 mEq sodium bicarbonate, and 1 bolus of 20% dextrose. She was started on an epinephrine infusion at 0.1 μg/kg/minute and transferred to the pediatric intensive care unit to receive an urgent hemodialysis (HD).
Fig. 1 depicts the trend of serum concentrations of lactate and metformin during hospitalization, with relation to ERTs. After 4 hours of HD (hour 7, day 1), pH increased from 6.80 to 7.17. However, lactate remained > 20 mmol/L despite adding norepinephrine (maximum rate, 0.18 μg/kg/minute) to the epinephrine infusion (maximum rate, 0.25 μg/kg/minute). Insufficient response to the dialysis prompted a transition to continuous venovenous hemodiafiltration (CVVHDF). After implementation of the CVVHDF (hour 11, day 1) and venoarterial ECMO (hour 20, day 1), lactate started to decrease, and eventually fell to normal range at around 48 hours. Of note, infusions of norepinephrine and epinephrine were discontinued at 30 and 79 hours, respectively.
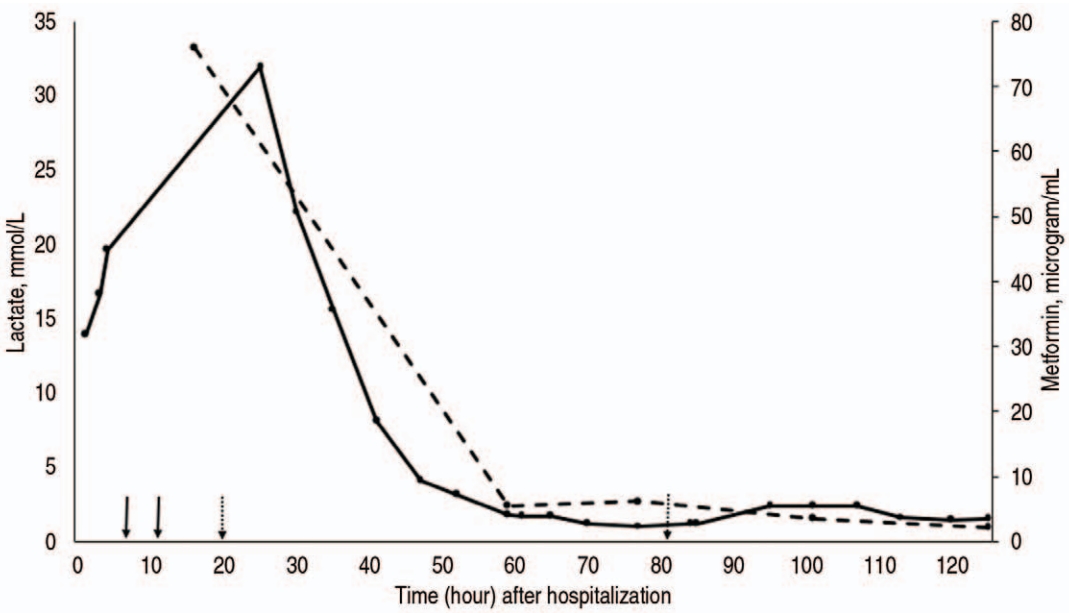
Trend of serum concentrations of lactate (solid line) and metformin (dashed line) during the hospitalization, with relation to extracorporeal removal techniques (solid arrows: initiation of hemodialysis and transition to continuous renal replacement therapy [from left to right]; dotted arrows: ECMO cannulation and decannulation). Ongoing lactic acidosis and shock prompted transition from hemodialysis (hour 7) to continuous venovenous hemodiafiltration (hour 11) and venoarterial ECMO (cannulation at hour 20, decannulation at hour 81). After the initiation of ECMO, lactate concentration began to downtrend and normalized around 48 hours. Norepinephrine and epinephrine were discontinued at hour 30 and 79, respectively. The peak concentration of lactate was 31.9 mmol/L, which was measured 17 hours after initiation of dialysis and 4 hours after initiation of ECMO. The peak concentration of metformin was 76 μg/mL, which was measured 8 hours after the initiation of dialysis. ECMO: extracorporeal membrane oxygenation.
As per medical records, the peak concentrations of lactate and metformin were 31.9 mmol/L and 76 μg/mL, respectively. True peak values were likely higher given the timing of blood-drawing and initiation of the ERTs. Notably, pH did not improve until initiation of the ECMO. This can be explained by continued lactate production combined with circulatory failure.
She was decannulated from ECMO on day 4 and extubated on day 14. Although CVVHDF was discontinued on day 9, she required daily HD through day 18. After transitioning to HD, blood urea nitrogen and creatinine initially rose to peak values of 159 and 6.3 mg/dL, respectively. She was cleared by psychiatry prior to discharge on day 25.
When the authors followed up with her family a few months later, her only residual symptom was neuropathy of the 1 toe. She has returned to school, and was participating on a sports team.
Discussion
A serum metformin concentration of > 5 μg/mL is associated with elevated lactate concentration and metabolic acidosis, with some studies claiming that a value of > 20-50 μg/mL suggests a worse prognosis (2,5). According to Dell’Aglio et al. (1), mortality is highest in patients with a pH nadir of < 6.9, peak lactate of > 25 mmol/L, or metformin of > 50 μg/mL (3,9). Importantly, serum metformin concentration is often not helpful in acute management largely due to a long turnaround time.
Metformin is water soluble and has limited protein binding, low molecular weight, and a high volume of distribution, which makes it moderately dialyzable (2,5,10). It is absorbed by the gastrointestinal system with a 50%-60% bioavailability, leading to the therapeutic concentration of 0.5-2 mg/L (3,5). When a metformin value is > 5 μg/mL, elimination is prolonged (3). It is 90% renally excreted and largely unchanged; a half-life in patients without renal impairment is 4-8.7 hours (2,5). Initiation of ERTs should be considered with a lactate of > 20 mmol/L, pH of < 7, shock, altered level of consciousness, or failure of standard supportive measures (2). The mainstay of dialysis is restoration of acid-base balance and removal of the metformin (4). Although HD is preferred due to more rapid clearance (HD, 200 mL/minute vs. continuous renal replacement therapy [CRRT], 50 mL/minute), hemodynamic instability secondary to acute overdose makes CRRT a superior ERT (2,10). In our case, we attempted HD first, but quickly pivoted to CVVHDF due to hemodynamic collapse.
Our team found several adult cases of metformin ingestions, but data in the pediatric population remain limited. One case series reported data of 55 children from 8 poison control centers, with metformin doses ranging from 9 to 196 mg/kg (11). In another study, no fatalities and minimal adverse effects were found in 1,546 patients younger than 19 years with reported metformin exposure (12). To our best knowledge, the present case represents the highest pediatric dose of ingestion, approaching the upper limit of the highest adult dose (144.5 g) (6,10,12-15).
The use of HD and CRRT for metformin overdose has been described, but the use of ECMO has been limited to case reports and series (16). We found 4 cases reporting the use of ECMO in adults who ingested metformin (17-20). The present case represents management of severe metformin ingestion in a pediatric patient with resultant cardiovascular collapse, successfully rescued with a combination of venoarterial ECMO and CVVHDF. Therapy for poisoning is largely supportive and symptomatic treatment, with ECMO being the most aggressive support modality offered (19). Bicarbonate is frequently used to correct acidosis but may result in worsening intracellular acidosis, sodium load, and a left shift of the oxyhemoglobin curve (2,5,10). Metformin has been associated with negative inotropic effects and decreased cardiac output, which can readily be supported with ECMO (4).
The use of ECMO for both intentional and unintentional ingestions has recently increased and demonstrated positive outcomes (8,19). In a review of cases reported in 2000-2018 National Poison Data System, 407 cases have used ECMO with 332 adults and 75 children, with a trend towards increased ECMO use over the study period. Survival was reported at 70%, which is consistent with other case series (8,19). This rising trend could represent increasing availability of ECMO over time (8). Our patient’s profound cardiovascular collapse led us to implement venoarterial ECMO, which ultimately enabled her to receive the dialysis required to clear the drug and correct the acidosis.
Timing of ECMO and defining its candidates should be further investigated given that ECMO has adverse events, is invasive, and requires significant resources (8,19). Our patient’s full recovery may support the role of early ECMO in patients presenting with acute overdose states requiring significant hemodynamic support.
Notes
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Funding sources
No funding source relevant to this article was reported.
Author contributions
Conceptualization, Data curation, Methodology, and Software: KL, LM, IA, and MD
Formal analysis and Investigation: all authors
Project administration and Supervision: MD
Validation and Visualization: KL and MD
Writing-original draft: KL, LM, IA, CYS, and MD
Writing-review and editing: all authors
All authors read and approved the final manuscript.
