Clinical features of children with drug poisoning in a single emergency department in Goyang, Korea
Article information
Abstract
Purpose
Pediatric drug poisoning is a frequent reason for emergency department (ED) visits. Considering the increasing number of mental illnesses in adolescents, it has become a serious public health problem. We aimed to investigate the drug poisoning in the ED.
Methods
We retrospectively analyzed children aged 1-17 years with diagnostic codes related to drug poisoning who visited the ED from January 1, 2010 through October 7, 2022. Exclusion criteria were non-pharmaceutical poisoning, insufficient data, and poisoning via respiratory, dermal or ocular route. Baseline characteristics, clinical manifestations, and outcomes of drug poisoning were analyzed in the study population, according to intention of poisoning and drug category.
Results
A total of 197 cases of 161 children were analyzed. Compared with non-intentional poisoning, intentional poisoning was associated a higher age, a longer time from ingestion to visit, and higher proportions of girls, antipyretics/analgesics or psychotropic drugs, symptoms related to the gastrointestinal, neurologic or cardiopulmonary systems (P = 0.034), psychiatric comorbidity, multiple drug ingestion, suicide attempt, decontamination (P = 0.017), the use of antidote, history of drug poisoning, and hospitalization (P = 0.004; all other Ps < 0.001). Acetaminophen, a representative of antipyretics/analgesics, was associated with a longer time from ingestion to visit and higher proportions of girls (P = 0.004), the presence of initial gastrointestinal symptoms, suicide attempt (P = 0.001), the use of antidote, and hospitalization (all other Ps < 0.001). Psychotropic drug was associated with higher proportions of psychiatric comorbidity (P < 0.001) and multiple drug ingestion (P = 0.012).
Conclusion
This study will enable pediatricians or emergency physicians to obtain an overview of the management of drug poisoning in EDs.
Introduction
According to the World Health Organization, acute poisoning is associated with approximately 45,000 annual deaths among patients younger than 20 years1). In Korea, 133,646 patients with the same age range visited health care facilities with the Korean Classification of Disease, 8th Revision codes related to drug poisoning (T36-65) annually from 2014 through 20182,3). In addition, suicide has been the most common cause of death among teenagers since 2009, with poisoning accounting for 49.2% of their suicidal methods4,5). It is important to understand the features of pediatric drug poisoning to provide appropriate emergency care. Thus, we aimed to analyze the baseline characteristics, clinical manifestations, and outcomes according to the intention of poisoning and drug category in children with drug poisoning who visited the emergency department (ED).
Methods
We reviewed electronic medical records of children aged 1-17 years with the aforementioned diagnostic codes related to drug poisoning (T36-65) who visited the ED of National Health Insurance Service Ilsan Hospital (Goyang, Korea) from January 1, 2010 through October 7, 20222). The ED staff cares for approximately 7,000 children annually. We excluded cases of non-pharmaceutical poisoning (e.g., exposure to household products), insufficient data or poisoning via respiratory, dermal or ocular route. Emergency practices for drug poisoning were performed by pediatricians on duty. Decontamination, such as orogastric lavage and administration of activated charcoal, was selectively performed considering the lapse of time from the ingestion (≤ 1-2 hours), airway protection, the degree of cooperation, and potential severity of poisoning. Two or more visits for drug poisoning of a single child were regarded as separate cases and as having history of drug poisoning from the second time. Those who ingested several types of drugs at once were regarded as multiple drug ingestion (DI). The ethics committee approval was obtained for the research on October 20, 2022 with a waiver of informed consent (IRB no. 2022-10-008).
In each case, age, sex, time from ingestion to visit (hours), intention of poisoning, drug category, initial manifestations, psychiatric comorbidities, multiple DI, suicide attempt, decontamination, antidote therapy, history of drug poisoning, and ED outcomes were investigated. The outcomes included hospitalization (overall and to the intensive care unit [ICU]), length of hospital stay (days), and in-hospital mortality. Laboratory data included aspartate and alanine aminotransferases, arterial blood gas analysis, and creatine kinase (CK).
Intentional drug poisoning was defined as an ingestion that was determined to be a willful overdose, as in a suicide attempt or gesture6). Non-intentional poisoning included cases in which drugs were mistaken for candies by infants and toddlers, taken by older children with mental retardation without recognition or given by caregivers without knowing the proper amounts.
The drug categories included antipyretics/analgesics, psychotropic drugs, antihistamines/cold preparations, and miscellaneous. In a case of multiple DI, the case was classified by a drug ingested at higher dose. In a category, all generic names or subcategories were recorded. In cases of acetaminophen (AAP) poisoning, N-acetylcysteine (NAC) was infused per a 21-hour intravenous (IV) protocol (a total of 300 mg/kg)7). Hepatocellular injury was defined as an alanine aminotransferase concentration greater than 2 times the upper normal limit. In cases of psychotropic drugs, elevated CK was defined as a CK concentration greater than 5 times the upper normal limit.
For continuous variables, Mann-Whitney U tests and Kruskal-Wallis H tests were used. For categorized variables, chi-square tests were used to analyze the difference between the groups. Statistical significance was defined as P < 0.05. All statistical analyses were done by SPSS software package for Windows ver. 22 (IBM Corp., Armonk, NY).
Results
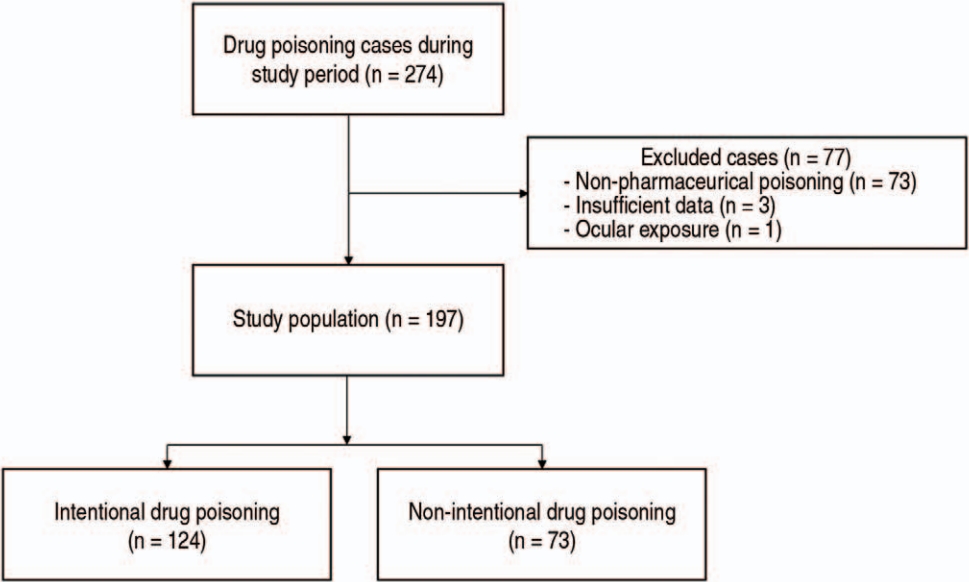
Among a total of 274 cases of drug poisoning, 77 were excluded (Fig. 1). Resultingly, 197 cases of 161 children were included, of which 124 (62.9%) were reported to be intentional. Seventeen patients showed 2-6 repeated episodes of poisoning, making a total of 36 cases with history of drug poisoning.
Table 1 shows that compared with non-intentional poisoning, intentional poisoning was associated with a higher age, a longer time from ingestion to visit, and higher proportions of girls, antipyretics/analgesics, psychotropic drugs, the presence of initial manifestations related to the gastrointestinal, neurologic or cardiopulmonary systems, psychiatric comorbidity, multiple DI, suicide attempt, decontamination, the use of antidote, history of drug poisoning, and hospitalization. No in-hospital mortality occurred in the population. In terms of the drug category, psychotropic drugs (67 [34.0%]) were most commonly taken, followed by antipyretics/analgesics (58 [29.4%]), miscellaneous (46 [23.4%]), and antihistamines/cold preparations (26 [13.2%]).

Clinical features of the children with drug poisoning who visited the emergency department, according to intention of poisoning (N = 197)
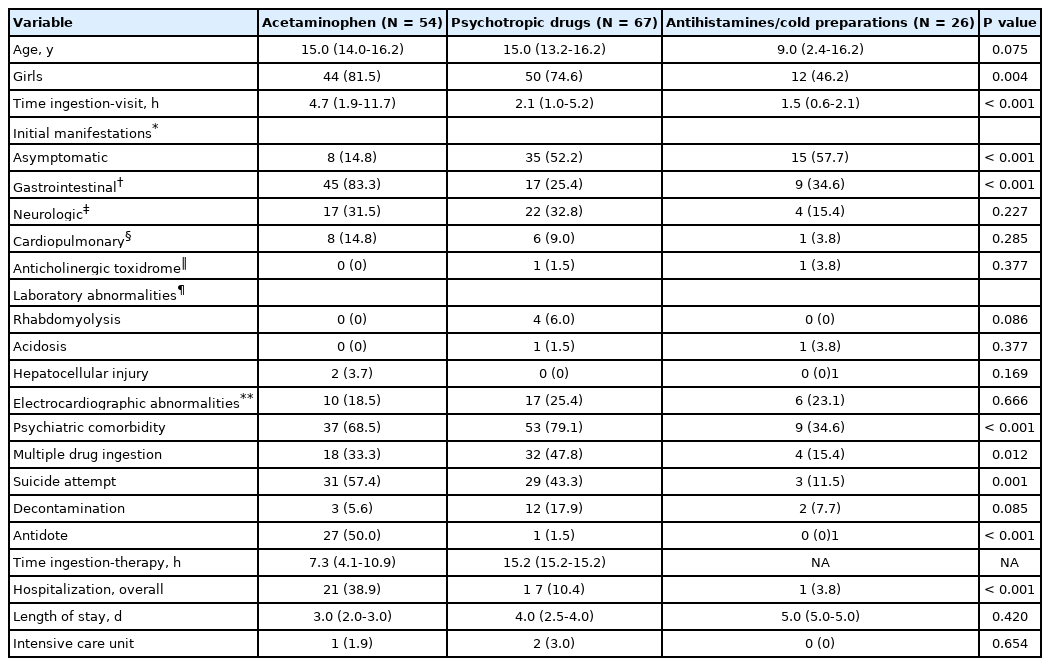
Clinical features according to the drug category are summarized in Table 2. Among the 58 cases of antipyretics/analgesics poisoning, 54 were AAP poisonings and the other 4 were ibuprofen poisoning with a median dose of 33.5 mg/kg (therapeutic dose range, < 28 mg/kg/day). Given that the 4 cases were asymptomatic, only the AAP cases were tabulated (Table 2). Among the categories, AAP poisoning was associated with a longer median time from ingestion to visit and higher proportions of girls, the presence of initial gastrointestinal manifestation, suicide attempt, the use of antidote, and hospitalization.
In all but 6 cases of AAP poisoning, the time from ingestion to visit was 24 hours or shorter. Median ingested dose and concentration were 109.7 mg/kg (interquartile range, 70.3-195.1 mg/kg) and 35.0 μg/mL (3.6-92.7 μg/mL), respectively. NAC was administered to 27 cases, including the ingestion of more than 150 mg/kg of AAP (n = 19), severe symptoms (n = 4), and co-ingestion of alcohol (n = 4). Two hepatocellular injuries were exclusively observed in the AAP poisonings. A 15-year-old girl with a hepatocellular injury showed a peak concentration of alanine aminotransferase of 3,306 IU/L and an international normalized ratio of 1.6. She was discharged uneventfully after NAC therapy and observation over 1 night in the ICU.
Psychotropic drug poisoning was associated with higher proportions of psychiatric comorbidity and multiple DI (Table 2). Thirty-two cases involved 2-5 psychotropic drugs simultaneously. Because of the variability of drugs involved, ingested quantity was calculated as the total number of tablets. The median number of tablets ingested was 20.0 (interquartile range, 8.0-33.5). Some of the neurologic symptoms, including tremors (n = 2), dystonia (n = 1), drooling (n = 1), and hiccups (n = 1), were only observed in the psychotropic drug poisoning. In all rhabdomyolysis cases, concentrations of CK were rapidly normalized with IV hydration, except in the case of a 15-year-old girl undergoing continuous renal replacement therapy. Although QTc prolongations were frequently seen in the psychotropic drug poisoning, all prolonged QTc were normalized on the day after electrolyte repletion or at the first outpatient visit. L-carnitine was administered as an antidote to another 15-year-old girl with valproic acid poisoning (587 mg/kg) whose initial concentration of valproate acid was higher than 300 μg/mL. Of the 2 ICU cases, besides the aforementioned girl undergoing continuous renal replacement therapy, a 6-year-old boy who was erroneously administered chloral hydrate of 460 mg/kg at a dental clinic developed respiratory distress with severe respiratory acidosis. He was discharged after 4-day mechanical ventilation.
Although the cases of antihistamines/cold preparations poisoning showed a higher rate of asymptomatic poisonings and lower rate of hospitalization than those of AAP poisoning, a 17-year-old girl who ingested diphenhydramine (50 mg/kg) had severe symptoms. She underwent a 1-minute generalized tonic-clonic seizure at the ED, and showed a lactic acid concentration of 16.0 mmol/L. Electrocardiography showed sinus tachycardia with a heart rate of 150 beats/minute. After administration of a single dose of IV lorazepam, repeated doses of sodium bicarbonate, and normal saline hydration, her laboratory findings improved, and she was discharged after 5 days.
The cases of miscellaneous poisoning contained most types of drugs that can be encountered at home (Table 3). Forty-one cases involved unintentional ingestions by preschoolers with minimal doses, causing no major clinical or laboratory abnormalities in most cases.
Discussion
This study shows the following findings. First, intentional poisoning was associated with adolescence (mostly girls), delayed visits, ingestion of antipyretics/analgesics or psychotropic drugs, symptomatic poisoning, psychiatric comorbidity, multiple DI, suicide attempt, emergency measures, history of drug poisoning, and hospitalization. Hospitalization was considered to have low severity given the absence of differences in the median length of stay, intensive care, and in-hospital mortality. Second, the features related to the intentional poisoning were also demonstrated in the poisoning of AAP or psychotropic drugs.
Girls’predominance was noted in both comparisons according to the intention and drug category. This finding is consistent with previously reported girls’predominance in adolescent poisoning1,8). Age distribution peaked at nearly 15.2 years in the intentional group and 2.1 years in the non-intentional group, which was also similar to other studies 9,10). The drug categories that were intentionally ingested consisted mostly of readily available or regularly taken drugs.
In this study, AAP poisoning showed the highest proportion of having gastrointestinal symptoms. In 83.3% of AAP poisoning, the children developed gastrointestinal symptoms, such as nausea and vomiting (n = 44), abdominal pain (n = 17), and dyspepsia (n = 2), which are similar to the previous study11). Since the NAC therapy is well known for the prevention of hepatotoxicity in AAP poisoning12), the rate of antidote use for AAP poisoning was higher than the other drug categories. This led to the highest hospitalization rate in AAP poisoning due to the 21-hour IV protocol of NAC. Apart from the administration of NAC within 24 hours of ingestion, IV NAC is recommended for patients with delayed presentation (> 24 hours) or evidence of hepatic injury13,14). The amount of ingested AAP and the lapse of time from the ingestion by 2 children with hepatocellular injuries were as follows: 209 mg/kg and 84 hours; and 148 mg/kg and 94 hours. Both children recovered with IV NAC despite the delayed presentation.
In the cases of psychotropic drugs poisoning, asymptomatic or discharge cases were more common compared to the cases of AAP poisoning because most of the ingested psychotropic drugs had a weak toxicity. Benzodiazepines (BZDs), selective serotonin reuptake inhibitors (SSRIs), and atypical antipsychotics were the 3 most common drugs ingested (Table 3). SSRIs have less severe toxicity than tricyclic antidepressants in overdose, and rarely result in fatality15). In this current study, a single case of tricyclic antidepressants poisoning was reported. A 1-year-old boy accidentally took the drugs within the therapeutic dose range, and remained asymptomatic. Although overdose of BZDs and atypical antipsychotics without other co-ingestions rarely cause a severe toxicity, BZDs can sometimes lead to central nervous system depression or hemodynamic instability16,17). In a case of poisoning by the psychotropic drugs, a 15-year-old girl developed loss of consciousness and respiratory distress. Flumazenil was not tried in the study population because of its short half-life, adverse effects such as convulsions18), and the on-duty pediatricians’ preference for conservative therapy.
This study has a few limitations. First, as the hospital is not a tertiary care center, some children requiring ICU care might have been initially transferred to a nearby tertiary hospital. This could explain the low incidence of morbidity and mortality in this current study. Second, since this study included cases of all kinds of drug poisoning, the detailed evaluation of single drug category may be lacking.
Notes
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Funding sources
No funding source relevant to this article was reported.