12세 남자 운동선수에서 2회 재발한 운동 유발 열사병 연관 횡문근융해증
Exertional heat stroke-related rhabdomyolysis recurring twice in a 12-year-old boy athlete
Article information
Trans Abstract
Exertional heat stroke (EHS) is a life-threatening entity characterized by elevated core temperature with potential for multiorgan dysfunction. EHS-related rhabdomyolysis usually occurs in the early phase. We report a boy athlete with EHS-related rhabdomyolysis, which recurred 15 and 79 days after the initial event. This case indicates that EHS can cause recurrences of rhabdomyolysis along with persistent symptoms, which could hamper the return to daily activity. Children with EHS, particularly athletes, may be at risk for recurrent rhabdomyolysis.
Introduction
Exertional heat stroke (EHS) is a medical emergency resulting from a rise in core temperature, where extreme production of heat overwhelms physiological heat dissipation due to high-intensity physical activity, particularly common in athletes and physical laborers1). Rhabdomyolysis, a complication of EHS, results from muscle necrosis with release of intracellular enzymes, such as creatine kinase (CK). Rhabdomyolysis is considered when serum CK concentration is greater than 5 times the upper normal limit or 1,000 IU/L2). EHS-related rhabdomyolysis is increasing in children with 5%-8% recurrence rates2).
We present a 12-year-old boy athlete who was successfully treated for EHS-related rhabdomyolysis, which showed a trimodal course. This study was approved by the institutional review board of Hanyang University College of Medicine (IRB no. 2021-12-040).
Case
A previously healthy 12-year-old boy, a baseball player, was brought to the emergency department (ED) with altered mental status in July 2021. He was not taking regular medications. On the day of the visit, he had been training outdoors for 2 hours with a 31.8°C maximum temperature and a 75.8% relative humidity. At the end of the session, which was 10 minutes prior to the visit, he had experienced nausea and syncope.
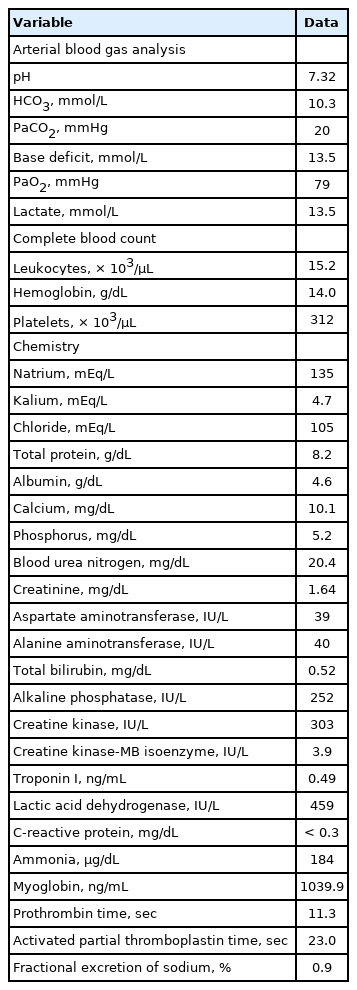
On arrival to the ED, the boy showed signs of shock, and the initial vital signs were as follows: blood pressure, 82/34 mmHg; heart rate, 171 beats per minute; respiratory rate, 26 breaths per minute; temperature, 41.8°C; oxygen saturation, 98% on room air; and a Glasgow Coma Scale of 7. His weight and height were 71 kg (> 97th percentile) and 165 cm (90-95th percentile), respectively. Initial laboratory findings showed compensated metabolic acidosis with pre-renal acute kidney injury (Table 1). With a presumptive diagnosis of EHS, we immediately cooled him by removing clothes, fanning for evaporative cooling, and applying ice packs to the whole body. We also provided him with oxygen via a reservoir bag and massive intravenous cold saline. No diffusion-restricted lesions were found on the initial magnetic resonance imaging, which was performed to delineate the EHS-induced ischemic stroke.
The trends of laboratory findings are summarized in Fig. 1. After hospitalization to the intensive care unit (7 hours after the first arrival to the ED), the Glasgow Coma Scale improved to 14. Nevertheless, he was still disoriented and did not recall the event. His tympanic temperature decreased to 38.3°C after the abovementioned cooling procedures. In addition, his urine output was maintained at more than 1 mL/kg/hour with the use of intravenous furosemide, and his serum creatinine improved to 1.2 mg/dL. However, subsequent laboratory findings suggested EHS-related rhabdomyolysis: CK, 3,030 IU/L (reference value, 5-130 IU/L; MM type, 98.2%); serum myoglobin, 1,039.9 ng/mL (25-72 ng/mL); and urine myoglobin, 222.40 ng/mL (< 21 ng/mL). On day 2, concentrations of CK and myoglobin were well-controlled with the administration of Ringer’s lactate solution and intravenous furosemide, resulting in the transfer to the ward. On day 10, the boy was discharged with serum CK of 100 IU/L and myoglobin of 36.4 ng/mL. Due to possible recurrence of rhabdomyolysis, he was recommended to refrain from exercise until the next follow-up visit.

The changes in the 12-year-old boy athlete’s serum concentrations of CK, myoglobin, AST, and ALT since the first arrival to the emergency department. The CK increased to 3,030 IU/L (+ 1 day), and normalized before the first discharge (104 IU/L) (+ 8 days). The second surge of CK above 20,000 IU/L was observed (+ 15 days), and that of 1,196 IU/L at the second discharge (+ 36 days). The third surge of CK occurred 50 days after the first arrival, peaking at 3,665 IU/L (+ 79 days). CK: creatine kinase, AST: aspartate aminotransferase, ALT: alanine aminotransferase.
Six days after the discharge (15 days after the first arrival to the ED), the boy visited the outpatient clinic. A couple of days before visiting the clinic, he went for a light walk of approximately 100 m, and later developed calf muscle cramps. Laboratory findings suggested a recurrence of rhabdomyolysis; serum CK, > 20,000 IU/L; and myoglobin, > 1,200 ng/mL (reached maximum concentrations before dilution). He was hospitalized again, and intravenous hydration was started immediately. Electromyography showed fibrillation potential and polyphasic motor unit action potential patterns, suggestive of myopathy. Aspartate and alanine aminotransferases peaked at 2,021 IU/L and 881 IU/L, respectively (16 and 22 days after the first arrival to the ED, respectively). These values returned to the normal range without an occurrence of hepatic failure before the second discharge. After supportive care, the boy was discharged with serum CK of 1,196 IU/L and creatinine of 0.54 mg/dL after 22 days (36 days after the first arrival to the ED). During the second hospitalization, no signs of metabolic myopathies were found in tandem mass spectrometry, plasma amino acid and acylcarnitine, urine organic acid, and whole exome sequencing.
Given the still high CK concentration and the need to prevent another recurrence, exercise was strongly prohibited at the second discharge. Two weeks after the discharge (50 days after the first arrival to the ED), we cautiously permitted exercise again after confirmation of CK normalization (104 IU/L). In subsequent laboratory tests in the outpatient clinic, CK began increasing up to 3,665 IU/L after he started to perform light intensity exercises, such as sit-ups and push-ups (79 days after the first arrival to the ED). After the limitation of exercise, the CK concentration was corrected without hydration, and the boy has been on the long-term observation with regular blood tests. The findings of myopathy improved on a follow-up electromyography (85 days after the first arrival to the ED).
Discussion
This case shows a trimodal course of EHS-related rhabdomyolysis, which recurred right after the light walk. Previous reports have shown bimodal course of EHS-related rhabdomyolysis, mostly in Asian countries. Yoshizawa et al.3) reported an adult with carnitine palmitoyltransferase II deficiency, myopathic form. Miura et al.4) and Ubaldo et al.5) reported cases of bimodal EHS-related rhabdomyolysis, which were eventually aggravated to hepatic failure. Unlike these cases, the current case featured the trimodal course without genetic or metabolic causes or hepatic failure. To our knowledge, this is the first pediatric case of a trimodal course of EHS-related rhabdomyolysis in Korea.
Reflecting on this case, athletes with EHS may be at risk of recurrent rhabdomyolysis. We hypothesized that the stress of the rehabilitation exercise, the 100-m walk, could repeatedly damage the weakened muscles. This hypothesis is supported by the following features. First, exercise increases the concentration of transforming growth factor-beta-16). Second, this cytokine contributes to fibrosis of the injured muscles, rendering the muscles vulnerable to further injury6,7). Third, this mechanism is exemplified by the fact that the premature return to physical activity by the boy’s motivation to be a professional player was followed by the recurrent episodes8).
It is noteworthy that the peak CK concentration was higher during the second hospitalization, and that there was the third increase in the concentration, i.e., a trimodal course. A previous study reported that CK usually normalizes over 8 days after the onset of rhabdomyolysis9). In our case, the CK normalized over more than 2 weeks at the second hospitalization. A similar pattern was noted in the concentrations of aminotransferases. EHS-related rhabdomyolysis and hepatic injury may be caused by hypoxia and ischemic injury10). It has not yet been established with respect to the mechanism of higher peak CK concentrations at the second event.
Currently, there are no evidence-based guidelines on the return to physical activity after EHS. However, we can reflect on the clinical guidelines proposed by the Consortium for Health and Military Performance11,12). For young athletes who experienced EHS-related rhabdomyolysis, confirmation of normal laboratory findings, such as CK, with light daily activity may precede regular activity. Then, gradual return to the latter with regular follow-ups can be tried.
This case indicates a potential for recurrent episodes of EHS-related rhabdomyolysis in pediatric athletes. This speculation may be considered at the initial presentation to EDs.
Notes
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Funding sources
No funding source relevant to this article was reported.