 |
 |
AbstractPurposeThe authors aimed to investigate the utility of blood culture (BC) for children with simple febrile seizure (SFS) in the emergency department (ED) in the post-10/13-valent pneumococcal conjugate vaccine (PCV) era.
MethodsThis study was performed at the ED of a tertiary care university-affiliated women and children’s hospital, and involved 3,237 previously healthy children aged 6-60 months who visited the ED with SFS from January 2013 through December 2017. The SFS was defined according to the International Classification of Diseases, 11th Revision codes related to seizure. The children were divided into 2 groups according to the vaccination rates of the period of their visit: the 70-PCV (70%, 2013-2014) and 97-PCV (97%, 2015-2017) groups. The primary outcome was the yield, defined as a true positivity of BC. In addition, we collected information on baseline characteristics, ED length of stay, inflammatory biomarkers, and ED outcomes.
ResultsOf the 1,578 children with SFS who underwent BC, 1,357 belonged to the 97-PCV group. The median age of the study population was 22 months (interquartile range, 16.0-30.0), and 935 children (59.3%) were boys. Of the 41 children (2.6%) with positive BC results, 3 had the yield (0.2%): Staphylococcus aureus in 2 children and Streptococcus pneumoniae in the other. All 3 children belonged to the 97-PCV group. There were 38 contaminated BCs (2.4%; 95% confidence interval, 1.6%-3.2%). The 97-PCV group showed a shorter median ED length of stay (166.0 minutes [108.0-279.5] vs. 143.0 [109.5-209.5]; P = 0.010) and a lower rate of hospitalization (39.4% vs. 12.8%; P < 0.001). No differences between the 2 groups were found in the baseline characteristics and biomarkers.
IntroductionFebrile seizure is more prevalent in Asian countries: 5%-10% in India, 6.9% in Korea, 3.4%-9.3% in Japan, and 14% in Guam, compared with the rate of 2%-5% in the United States and Europe [1-4]. In emergency departments (EDs), blood culture (BC) has been frequently performed as an initial workup for febrile children to identify occult bacteremia (OB). For this clinical purpose, BC is still routinely performed in children with simple febrile seizure (SFS) despite the low-quality evidence on BCs as per the American Academy of Pediatrics guidelines for SFS [5].
Rates of OB had changed in febrile children depending on the serotype coverage of pneumococcal conjugate vaccine (PCV). In the post-7-valent PCV era, the rate ranged from 0.3% to 0.9% [6-10]. Also, the rate was 0.02%-0.2% in the post-13-valent PCV era [11,12]. A Japanese study showed a decrease in the rate in children with febrile seizure after 2010, point of licensing of the 10- and 13-valent PCVs [13].
The authors aimed to investigate the utility of BC in children with SFS who visited the ED of a tertiary care hospital with the yield, defined as a true positive result of BC.
Methods1. Study design and settingThis retrospective study was conducted at the ED of CHA Bundang Medical Center in Seongnam, Korea, with annual visit of approximately 25,000 children. The authors reviewed previously healthy children aged 6-60 months with SFS who visited the ED from January 2013 through December 2017. We investigated the yield as the primary outcome, in addition to the baseline characteristics, ED length of stay (EDLOS), inflammatory biomarkers, and ED outcomes in the post-10/13-valent PCV era. A sub-analysis was performed to study the relevant differences between the period 2013-2014, which showed a 70% vaccination rate (“70-PCV group”), and 2015-2017, which showed a 97% vaccination rate (“97-PCV group”) [14,15]. In Korea, 10- and 13-valent PCVs were licensed in 2010 and included in the national immunization program in 2015 [16]. The study protocol was approved by the institutional review board of CHA Bundang Medical Center with a waiver for informed consent (IRB no. 2020-05-010).
2. Inclusion criteria and definitionsFebrile seizure was defined according to the International Classification of Diseases, 11th Revision codes related to seizure (R56.0-R56.8). Children were included if they were diagnosed with SFS, and had undergone BC during the initial evaluation at the ED. The exclusion criteria were as follows: misdiagnosis, no BC, and presence of underlying medical conditions, such as central nervous system diseases predisposing children to seizures, presence of cardiopulmonary diseases, and an immunocompromised status.
3. Performance and interpretation of BCBC was performed using Bact/ALERT 3D (bioMérieux, Grasse, France). During the study period, BCs were obtained by ED physicians or nurses using sterile techniques, and the samples were inoculated into a pair of aerobic and anaerobic bottles. We only included children with 1 mL or more blood samples inoculated into each bottle.
Pathogenic bacteria were defined as Streptococcus pneumoniae, Staphylococcus aureus, group A beta-hemolytic streptococci, and Haemophilus influenzae. Contaminants were defined as one of the following: coagulase-negative staphylococci, viridans group streptococci, Bacillus spp., Micrococcus spp., Propionibacterium spp., and Corynebacterium spp. [17,18] If no growth was reported after 5 days of BC, it was considered negative.
4. Data collectionStandardized data collection sheets were used. Along with the BC results, we collected information on the baseline characteristics, including age (months), sex, initial temperature and oxyhemoglobin saturation, recent use of antibiotics (< 3 days), and EDLOS (minutes), inflammatory biomarkers (white blood cell count and C-reactive protein concentration), and ED outcomes (discharge, hospitalization, transfer, death, and discharge against medical advice).
5. Statistical analysisStudent t-tests or Mann-Whitney U-tests were performed for continuous variables, and chi-square tests or Fisher exact tests were used for categorical ones. Statistical significance was set at P < 0.05. Statistical analysis was performed using IBM SPSS for Windows ver. 26.0 (IBM Corp., Armonk, NY).
Results1. Study populationOf 3,237 eligible children, 1,578 were enrolled (Fig. 1). Table 1 outlines the clinical characteristics of the study population. No differences between the 2 groups were found with respect to the median age, rates of boys and recent use of antibiotics, initial temperature and oxyhemoglobin saturation, and values of the biomarkers. The median EDLOS and the rate of hospitalization were respectively shorter and lower in the 97-PVC group than in the other group.
2. Positive BC resultsAmong the 1,578 children, 41 (2.6%; 95% confidence interval, 1.9%-3.0%) had positive BC results (Table 1). Of these 41 children, 39 (2.5%) belonged to the 97-PCV group. Three children had the yield (0.2%; 0%-0.3%) that included S. aureus and S. pneumoniae. All 3 children belonged to the 97-PCV group were discharged without antibiotics, and were lost in follow-ups without undergoing the second BCs (Table 2). The other 38 children had contaminants (overall contamination rate, 2.4%; 1.6%-3.2%).
DiscussionThis study shows a 0.2% OB rate in the previously healthy children with SFS who visited the ED in the post-10/13-valent PCV era, indicating the low utility of BC in identifying OB in the era. This study suggests that the routine performance of BC in previously healthy children with SFS can lead to unnecessary economic loss and excessive blood sampling.
All 3 children with the yield belonged to the 97-PCV group. Although the vaccination status of the boy with S. pneumoniae OB was unknown, he was presumed to be immunized with 10/13-valent PCV given the contemporary vaccination rate of 97% (Table 2) [15]. The most common yield was S. aureus, which is consistent with a Californian study showing an increase in the proportion of S. aureus along with a decrease in that of S. pneumoniae among the bacteremic pathogens in post-10/13-valent PCV era [11].
There were no significant differences in the values of inflammatory biomarkers and in the ratio of positive BC results between the 70-PCV and 97-PCV groups. Complete blood count and BC are not diagnostic of bacteremia in children with SFS because the prevalence of invasive bacterial infection is extremely low among vaccinated children [19]. As a result of this low prevalence, BC and empirical antibiotic therapy are no longer recommended, particularly in previously healthy children with SFS. The low yield in our study suggests the need to refine the current guidelines for obtaining BC in EDs with an emphasis on the risk factors of bacteremia rather than the severity of SFS.
On the other hand, a recent Japanese study showed a 52.7% (58 of the 110 children) rate of febrile seizure in the children aged 3-36 months with OB from 2002 through 2015, suggesting febrile seizure as a potential predictor of OB [13]. This Japanese study is also applicable to Korean children considering that the incidence of febrile seizure in Korea is higher than that in the U.S. and Europe [3]. Hence, BC may be needed in children with febrile seizure who have risk factors for OB, such as unvaccination or complex febrile seizure.
This study has some limitations. First, given the single-center design, the findings were potentially biased, and might not be easily extrapolated to other populations. Second, the vaccination status was estimated base on domestic statistics because of the lack of relevant records [14,15]. Third, given the estimated rate of BC performance of 48.7% (1,578 of the 3,237 eligible children), we could not report OB rate in the entire children with SFS. Fourth, the size of the 70-PCV group was smaller than that of the other group. This gap in the group sizes might stem from increasing visits to our center after the Korean government had designated it as a regional emergency medical center and a pediatric emergency center, and from the presumably lower performance of BC in children with SFS of the 70-PCV group. Fifth, the yield might be affected by the rate of recent antibiotic therapy (12.5%), which did not differ between the 2 groups.
In summary, we found a low utility of BC in identifying OB among previously healthy children with SFS in EDs in the post-10/13-valent PCV era. This study encourages the refinement of current guidelines for obtaining BC from children with febrile seizure in EDs in the era.
Fig. 1.Flowchart for the selection of study population. SFS: simple febrile seizure, ED: emergency department, PCV: pneumococcal conjugate vaccine. 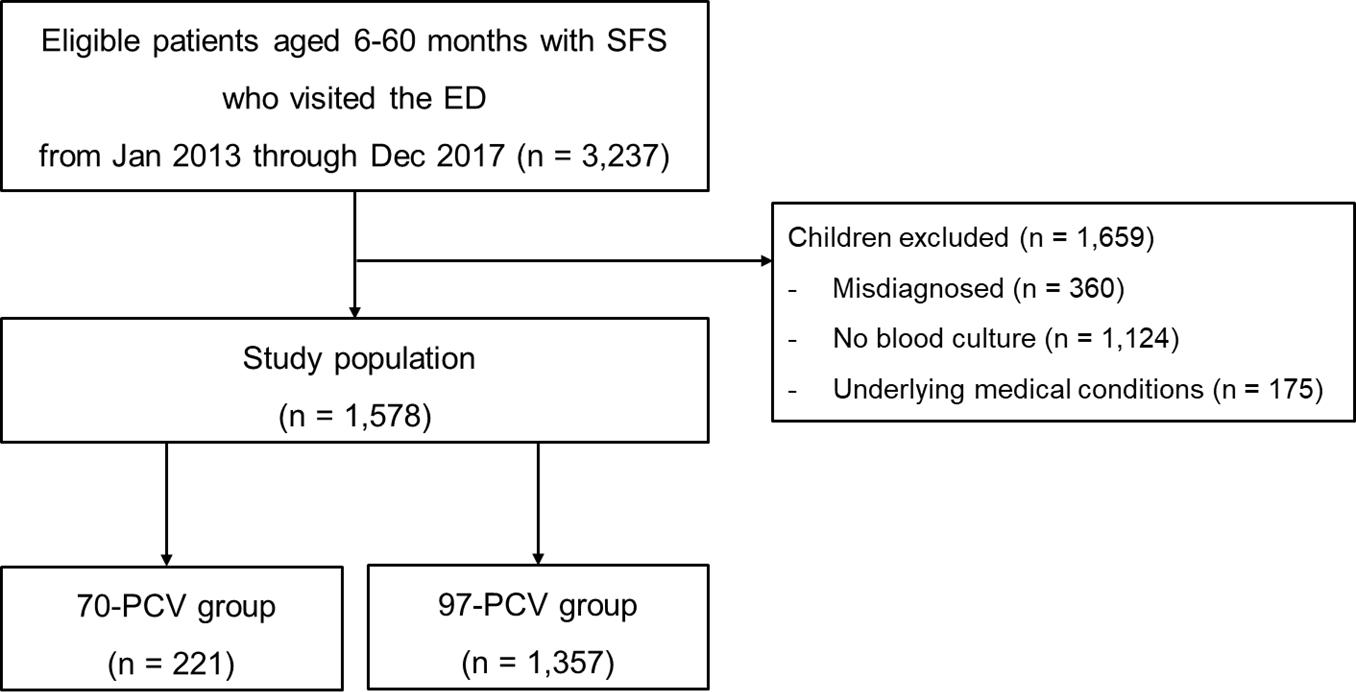
Table 1.Clinical characteristics of the study population Table 2.Microbiology and outcomes of the children with the yield
References2. Paul SP, Blaikley S, Chinthapalli R. Clinical update: febrile convulsion in childhood. Community Pract 2012;85:36–8.
3. Byeon JH, Kim GH, Eun BL. Prevalence, incidence, and recurrence of febrile seizures in Korean children based on national registry data. J Clin Neurol 2018;14:43–7.
4. Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures American Academy of Pediatrics. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics 2008;121:1281–6.
5. Subcommittee on Febrile Seizures; American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics 2011;127:389–94.
6. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children’s Hospital Emergency Department and Urgent Care Center. Arch Pediatr Adolesc Med 2004;158:671–5.
7. Sard B, Bailey MC, Vinci R. An analysis of pediatric blood cultures in the postpneumococcal conjugate vaccine era in a community hospital emergency department. Pediatr Emerg Care 2006;22:295–300.
8. Wilkinson M, Bulloch B, Smith M. Prevalence of occult bacteremia in children aged 3 to 36 months presenting to the emergency department with fever in the postpneumococcal conjugate vaccine era. Acad Emerg Med 2009;16:220–5.
9. Bressan S, Berlese P, Mion T, Masiero S, Cavallaro A, Da Dalt L. Bacteremia in feverish children presenting to the emergency department: a retrospective study and literature review. Acta Paediatr 2012;101:271–7.
10. Herz AM, Greenhow TL, Alcantara J, Hansen J, Baxter RP, Black SB, et al. Changing epidemiology of outpatient bacteremia in 3- to 36-month-old children after the introduction of the heptavalent-conjugated pneumococcal vaccine. Pediatr Infect Dis J 2006;April 25; 293–300.
11. Greenhow TL, Hung YY, Herz A. Bacteremia in Children 3 to 36 Months Old After Introduction of Conjugated Pneumococcal Vaccines. Pediatrics 2017;139:e20162098.
12. Leibovitz E, David N, Ribitzky-Eisner H, Abo Madegam M, Abuabed S, Chodick G, et al. The epidemiologic, microbiologic and clinical picture of bacteremia among febrile infants and young children managed as outpatients at the emergency room, before and after initiation of the routine anti-pneumococcal immunization. Int J Environ Res Public Health 2016;13:723.
13. Kamidani S, Shoji K, Ogawa E, Funaki T, Mishina H, Miyairi I. High rate of febrile seizures in Japanese children with occult bacteremia. Pediatr Emerg Care 2020;36:e199–203.
14. Korea Centers for Disease Control and Prevention (KCDC). Korea National Immunization Survey, 2013 [Internet]. Cheongju (Korea): KCDC: c2013 [cited 2017 Jul 27]. Available from: https://nip.kdca.go.kr/irgd/reference.do?MnLv1=7. Korean.
15. Korea Centers for Disease Control and Prevention (KCDC). National childhood vaccination coverage among children aged 1-3 and 6 years in Korea, 2018 [Internet]. Cheongju (Korea): KCDC: c2018 [cited 2019 Jul 31]. Available from: https://nip.kdca.go.kr/irgd/reference.do?MnLv1=7. Korean.
16. Korea Centers for Disease Control and Prevention (KCDC). Epidemiology and management of infectious diseases subject to vaccination [Internet] 5th ed. Cheongju (Korea): KCDC: c2017 [cited 2017 May 17]. Available from: https://nip.kdca.go.kr/irgd/reference.do?MnLv1=2. Korean.
17. Weinstein MP. Blood culture contamination: persisting problems and partial progress. J Clin Microbiol 2003;41:2275–8.
18. Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer lg, Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 1997;24:584–602.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |