Introduction
Coronary artery aneurysm (CAA) is a life-threatening complication of Kawasaki disease (KD). Thrombosis in KD with CAA may lead to morbidity and mortality, such as myocardial infarction[1]. Therefore, optimal treatment of children with KD with CAA, is crucial for thromboprophylaxis. Depending on the size of the aneurysm, second antiplatelet agents or anticoagulants may be added to aspirin therapy[2].
We describe a 6-year-old girl who was treated with warfarin after she had developed multiple, long, and medium-sized CAAs in the left anterior descending artery (LAD) and long segments of irregular dilatation and tortuosity in the right coronary artery (RCA). The girl showed a refractory response to conventional treatment of KD and adjunctive steroid therapy, and was at a risk of thrombosis due to turbulent flow inside the CAAs.
Case
The patient was a previously healthy, 6-year old girl. In August 2017, she was hospitalized at a hospital for fever that lasted for 7 days, conjunctival injection without any discharge, red lips and tongue, enlarged cervical lymph node, truncal maculopapular polymorphous rash, and edematous changes on both palms and soles. She was treated with intravenous immunoglobulin (2 g/kg) as the standard treatment protocol for KD.
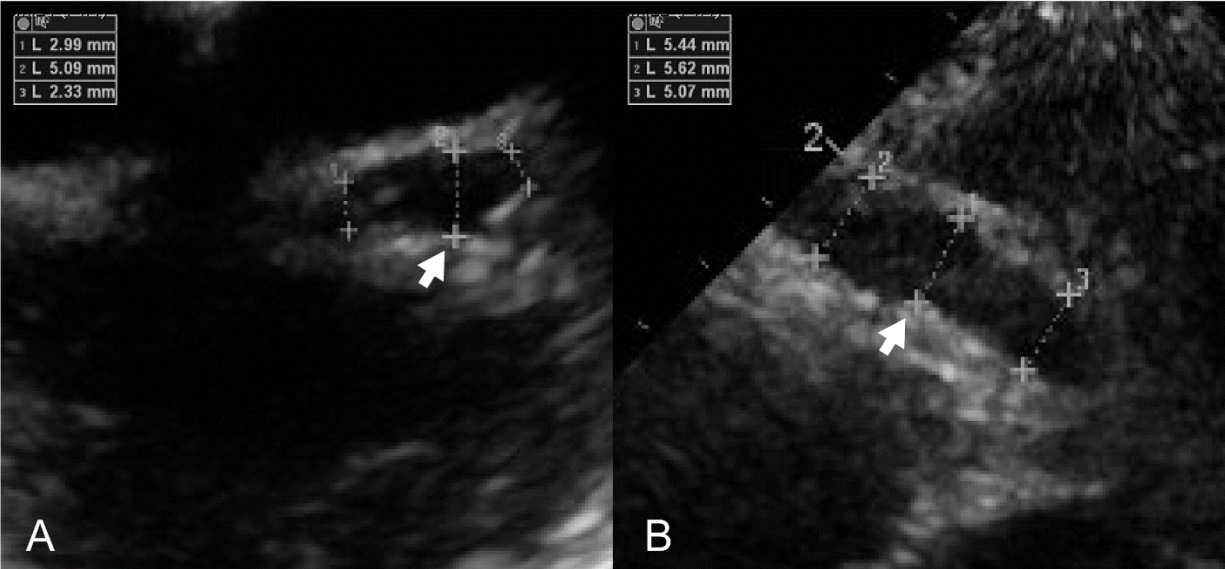
Despite the therapy, her symptoms waxed and waned. Echocardiography showed diffuse coronary artery ectasia of the left main coronary artery, an aneurysm of the LAD, and an irregular fusiform aneurysm and tortuous dilatation of the RCA (Fig. 1). Given these multiple CAAs, she was then referred to our hospital, and hospitalized to the general ward. The initial vital signs were as follows: blood pressure, 121/71 mmHg; heart rate, 88 beats per minute; temperature, 36.5В°C; and alert mentality. Her weight was 21 kg (25-50 percentile).
Initial laboratory findings showed a C-reactive protein concentration of 27.0 mg/L and procalcitonin concentration of 0.67 ng/mL (reference value, 0.02-0.5 ng/mL). Other laboratory findings were as follows: white blood cell count, 6.40 Г— 103/ОјL; hemoglobin, 10.8 g/dL; hematocrit, 31.5%; platelet, 488 Г— 103/ОјL; serum sodium, 136 mmol/L; potassium, 4.2 mmol/L; aspartate aminotransferase, 30 IU/L; alanine aminotransferase, 72 IU/L; albumin, 3.0 g/dL; total bilirubin, 0.5 mg/dL; and B-type natriuretic peptide, 218.4 pg/mL.
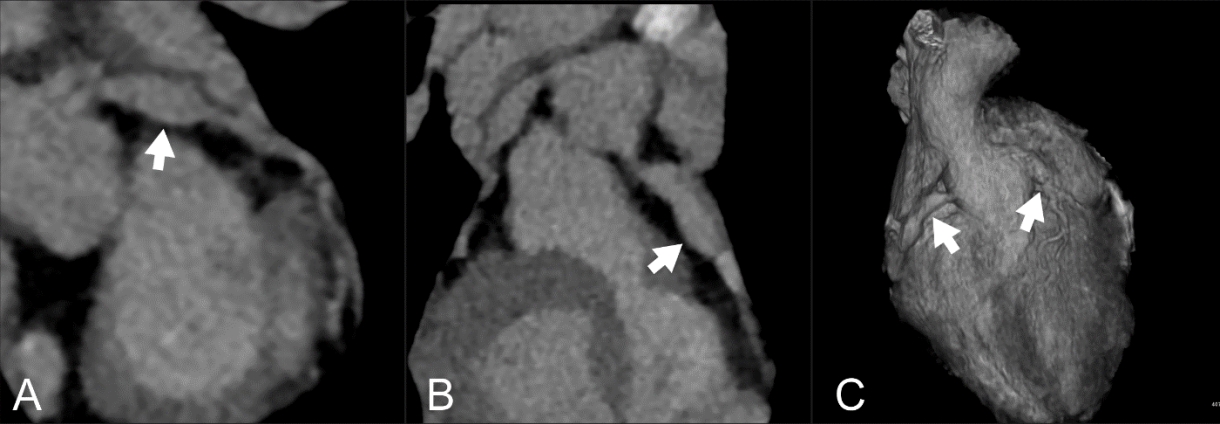
Adjunctive steroid therapy was performed in addition to intravenous immunoglobulin and aspirin, and no further manifestations of KD were noted. However, echocardiography showed coronary arteriopathy with aneurysms (spindle-shaped LAD ectasia of 5-6 mm [Z-score = 5.2] and RCA ectasia of 4-6 mm [Z-score = 5.1]). Cardiac computed tomography for evaluation of the distal coronary arteries showed aneurysms in multiple coronary segments, which were wider and longer than those reported in the echocardiography performed at the referring hospital (Fig. 2).
As per the 2017 American Heart Association guidelines, KD with medium-sized CAAs does not always require the administration of low-molecular-weight heparin or warfarin as thromboprophylaxis[3]. However, the anatomical features of the CAAs in our patient could cause turbulent blood flow inside the lesions, which is mentioned as a risk factor for myocardial ischemia in the guidelines[3]. Thus, we initiated long-term anticoagulation therapy with warfarin (2.5 mg/day). International normalized ratio (INR) remained within the target of 2-4 for 5 consecutive days without acute bleeding. The concentrations of C-reactive protein and procalcitonin normalized, and initial blood culture showed no bacterial growth. On day 7, she was discharged without residual manifestations of KD.
After 3 days, she visited the emergency department (ED) for mild fever and erythematous skin rash on her back, neck, and both legs. Laboratory tests related to KD showed normal results. In addition, the rash recurred on the same site as before, and disappeared spontaneously. She was discharged without measuring INR because the rash was not considered to be due to a potential coagulopathy.
Three days after the ED visit, she returned to the outpatient department with evident petechiae. Given a follow-up INR of 6.28, we concluded that it was risky to continue warfarin therapy. After discontinuing the therapy, her INR normalized within 2 days. Given her unusually high INR with the dose of warfarin, we performed genetic assays for warfarin-related genes to investigate the causes. The assays showed that her CYP2C9 genotype was *1/*3 (intermediate metabolizer) and her vitamin K epoxide reductase complex 1 (VKORC1; вҲ’1639G>A) genotype was AA. Because there are no dose-adjustment guidelines for children with genetic warfarin sensitivity, we decided to replace warfarin with clopidogrel.
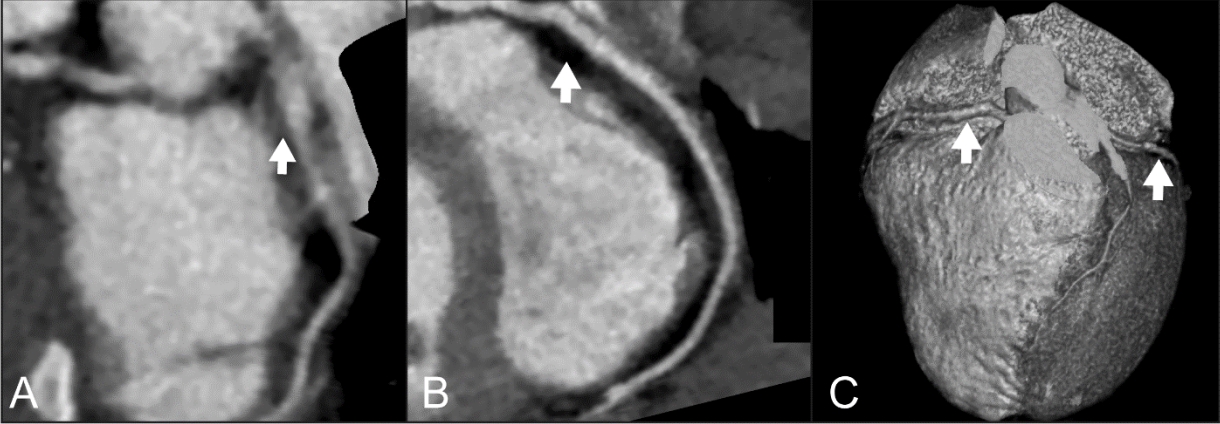
Follow-up echocardiography every 3 months showed no further dilatation or thrombosis of the CAAs. Moreover, a 29-month follow-up cardiac computed tomography (January 2020) showed significant improvement of the RCA and LAD lesions, even with residual mild dilatation and focal dilatation of the left circumflex artery (Fig. 3).
Discussion
This case report shows clinical implications of the genetic warfarin sensitivity in settings of anticoagulation for KD with CAA, and the potential for bleeding in cases of delayed detection of the sensitivity. Two previous articles have shown that dose adjustment and longer monitoring of INR are necessary in warfarin therapy for children with KD and associated warfarin sensitivity[4,5].
CYP2C9 has 3 genetic variants, including wild-type CYP2C9 *1, and variants *2 and *3. Variants *2 and *3 reduce the enzymatic activity, and result in relative sensitivity to warfarin compared to wild-type CYP2C9 *1. The VKORC1 gene, another major gene of warfarin sensitivity, encodes the vitamin K epoxide reductase enzyme that promotes vitamin K recycling. A common non-coding variant, вҲ’1639G>A, may increase warfarin sensitivity. In вҲ’1639A carriers who need warfarin therapy, initial and maintenance doses should be lower than in вҲ’1639G carriers[6].
For children with variant alleles, such as CYP2C9 *1/*3, *2/*2, *2/*3, *3/*3, VKORC1 GA, or AA, it takes longer to reach the maximum INR (about 2-4 weeks after the first warfarin administration)[6,7]. When reached, INR is higher in children with the variants than in those with the wild-type gene[6,7]. Therefore, in cases of children with genetic variants affecting warfarin metabolism, a hospital length of stay of 3-5 days for warfarin maintenance may be too short to prevent iatrogenic coagulopathy, resulting in unexpected return visits to EDs with bleeding. Given that the genotype-based, therapeutic dose recommendations for warfarin are available for adults, further studies are needed to determine pediatric guidelines[6].
Furthermore, because KD is characterized by polymorphic erythematous skin lesions, in children with recently diagnosed KD, coagulopathy-induced petechiae can be misdiagnosed as improving, incompletely treated or refractory KD. Hence, it is important to distinguish KD from coagulopathy. In typical KD, polymorphic rash is followed by desquamation[8], and erythematous changes in the extremities and at the Bacille Calmette-GuГ©rin inoculation sites are common[9]. In coagulopathy, petechiae do not blanch under pressure. If these differences are not recognized, there may be a delay in detecting life-threatening bleeding. Thus, this case suggests that history of recent anticoagulation needs to be obtained in EDs.
In conclusion, this case suggests that iatrogenic coagulopathy needs to be monitored in children with KD associated with CAA who have undergone recent anticoagulation. It is vital to obtain the relevant history, and if available, genetic assays for warfarin sensitivity in the children. To prevent not only thrombosis but also bleeding, regular monitoring of INR is mandatory until the confirmation of warfarin maintenance doses.