 |
 |
AbstractFactor VII deficiency is a rare, inherited coagulopathy, which can lead to prolonged bleeding. Here, we present a case report of an adolescent with factor VII deficiency who experienced small bowel bleeding that was successfully treated with tranexamic acid. This case highlights the potential use of tranexamic acid as an effective therapeutic option for managing gastrointestinal bleeding in patients with hemostatic insufficiency of unknown etiology.
IntroductionPediatric gastrointestinal bleeding (GIB) is a challenging clinical situation that requires establishing management principles based on rapid differential diagnosis, which differs from that in adults. Congenital coagulopathy can cause various bleeding symptoms and manifest as GIB. As per 2010-2015 Korean Health Insurance Review and Assessment Service database, the overall incidence of congenital coagulopathies was 0.78/100,000 person-years, with hemophilia A (38%), von Willebrand disease (25%), and hemophilia B (7%) as the top 3 diagnoses (1). The prevalence of factor VII deficiency is reported to be 300,000-500,000 people worldwide, and it accounts for 1.8% (45/2,458) of patients with congenital coagulation disorders in Korea, according to 2019 registration data of the Korean Hemophilia Foundation (2). The universal treatment of bleeding is based on supplementation of deficient factors, but there are no comprehensive clinical guidelines for GIB in undiagnosed cases.
Tranexamic acid (TXA), an antifibrinolytic agent, has limited efficacy for GIB. However, given its potential as a universal hemostatic agent, it has been evaluated for various indications, such as perioperative bleeding in patients with congenital coagulopathy (3). We report a case of GIB in an adolescent boy who was found to have factor VII deficiency. Through this, we carefully suggest the potential utility of TXA as an empirical hemostatic agent for refractory GIB. This study was reviewed by the institutional review board of Ajou University School of Medicine, and the requirement to obtain written informed consent was waived (IRB no. EX-2023-320).
CaseA previously healthy 17-year-old boy presented to our emergency department (ED) with a 2-day history of painless hematochezia (3-4 times, in total > 100 mL) with accompanied dizziness. He had no recent infection. Initial vital signs were as follows: blood pressure, 108/70 mmHg; heart rate, 86 beats/minute; respiratory rate, 12 breaths/minute; temperature, 36 ¬įC; oxygen saturation, 100%; and alert mentality. On initial physical examination, we noted pale conjunctivae and abdominal tenderness with normoactive bowel sound. No abnormalities were found on rectal and neurological examinations.
In the ED, the boy experienced 2 episodes of syncope, each lasting 5 seconds with pallor. In a standing posture, he showed the third syncope with a 114/61 mmHg blood pressure and 130 beats/minute heart rate, and received a 500-mL bolus of 0.9% saline. Complete blood count and coagulation battery were as follows: white blood cells, 7,000/őľL; hemoglobin, 13.1 g/dL; platelets, 184,000/őľL; prothrombin time, 14.4 seconds; international normalized ratio, 1.18; and activated partial thromboplastin time, 32 seconds. At that time, no transfusion was performed given the lack of anemia and possibility of obscuring underlying coagulopathy by transfusion. The boy passed an additional 300 mL of hematochezia without pain, and underwent an abdominal computed tomography, a non-dynamic contrast-enhanced routine phase, showing nonspecific findings. To further investigate the hemorrhage, upper and lower endoscopies were immediately performed, revealing persistent bleeding with a clot on the latter endoscopy (Fig. 1).
On day 2, a negative finding was noted on Meckel scan. On day 4, although his vital signs were stabilized, his hemoglobin dropped to 7.9 g/dL. Subsequently, capsule endoscopy showed bleeding from the terminal ileum to the colon, with a focus masked by blood on the mucosa (Fig. 2). Once a proximal small bowel bleeding was ruled out, we performed a follow-up colonoscopy to localize the persistent bleeding. The colonoscopy confirmed delayed hemostasis after endoscopic biopsy (Fig. 3). On the same day, a follow-up coagulation battery showed an international normalized ratio of 1.41 with activated partial thromboplastin time of 40 seconds. Despite this finding suggestive of coagulopathy, along with the persistent findings of obscure GIB on the follow-up colonoscopy, it remains uncertain which clotting factor is specifically deficient. Thus, the boy was administered TXA orally (12.5 mg/kg/day divided into 3 doses) without undergoing transfusion. On day 6, the gross hematochezia disappeared, and hemoglobin concentration rebounded to 8.8 g/dL (Fig. 4).
Eventually, an in-house hematologist diagnosed factor VII deficiency based on the following findings: factor V, 119.0% (reference value, 81%-160%); factor VII, 37.1% (68%-149%); and platelet function test, 139 seconds (61-116 seconds); ristocetin cofactor assay, 118% (56%-187%); and von Willebrand factor antigen, 131.8% (66.1%-197%). On day 9, the boy was discharged with an outpatient follow-up plan, and was found to have no residual bleeding-related symptoms.
DiscussionObscure GIB is a bleeding whose cause is not identified by primary evaluation, such as upper and lower endoscopies. The GIB accounts for 10%-20% of all-age GIB cases, with an approximately 50% recurrence rate (4). Bleeding from the small bowel not investigated by upper or lower endoscopy is the most common cause of obscure GIB. Several studies have demonstrated the validity of small bowel bleeding assessment tools, including capsule endoscopy and magnetic resonance enterography. In adults, the possibility of vascular dysplasia and intestinal tumors is given priority (5). This contrasts with a higher incidence of Meckel’s diverticulum and polyps in children (6). Various congenital coagulopathies can lead to GIB, particularly obscure GIB (7). This feature may be more important in children given the congenital nature of such coagulopathies. However, there were no diagnostic guidelines for coagulopathy-associated bleeding using conventional tools for small bowel bleeding (8).
Factor VII deficiency is a rare genetic disorder that features a defect in the production of the clotting factor caused by a mutation in the F7 gene on chromosome 23, inherited in an autosomal recessive manner (9), with a global prevalence of 1 in 500,000 (10). The clinical presentation is variable among individuals, with spontaneous bleeding and mild hemostatic disorders being most common, and one-third of affected patients remaining asymptomatic during their lifetime. The most common bleeding manifestations are gingival bleeding and menorrhagia. Hemarthrosis, intracranial hemorrhage, and GIB occur in 10%-15% and can be life-threatening (10). Factor VII deficiency is divided into type 1 (quantitative), and type 2 (qualitative), without a direct correlation between factor VII concentration and clinical severity. Existing literature suggests that factor VII concentration above 20% may serve as a preventive measure against spontaneous bleeding (11). However, the case patient experienced a severe clinical course of bleeding that lasted for several days, despite the concentration above 20% (37.1%).
TXA is a synthetic lysine that inhibits fibrinolysis, specifically by impeding the conversion of plasminogen to plasmin and by binding circulating plasmin to fibrin (3). The drug has been clinically as a hypothetical hemostatic agent in fibrinolysis-associated bleeding. After its usefulness was proven in severe trauma, such as trauma experiencing extracranial hemorrhage in 2010 (12) and postpartum hemorrhage in 2017 (13), TXA has been extensively evaluated as a universal hemostatic agent in situations without overt evidence of fibrinolysis. Concerns remain about the adverse events, such as seizures and thromboembolism (3), and limited results on mortality benefits (14). However, its efficacy has been documented in reducing tooth extraction bleeding, preventing short-term hemorrhage in children with hemophilia, and controlling heavy menorrhagia, as well as in trauma and postpartum hemorrhage (3).
The role of TXA in GIB is controversial. A meta-analysis of 13,000 cases in 8 studies found no increase in rebleeding, need for surgery, or adverse events on treating GIB with TXA (15,16). It has been suggested that TXA is effective in preventing intraoperative bleeding in patients with von Willebrand disease, and useful for hemostasis in those with coagulopathies, leading to the tentative recommendation of antifibrinolytic therapy for coagulopathy-associated GIB (17,18).
We report an adolescent case of GIB due to factor VII deficiency treated with TXA in the context of a complex diagnosis and unclear outcome. While the utility of TXA as a universal hemostatic agent requires further validation, TXA could serve as a hemostatic agent in various bleeding scenarios related to fibrinolysis needing prompt therapeutic intervention, such as pediatric GIB without a focus. Therefore, the role of TXA warrants reevaluation, extending beyond its use in specific conditions and as a secondary therapeutic option in EDs.
NotesAuthor contributions Conceptualization, Formal analysis, Methodology, Project administration, and Resources: YB Kim Data curation: KH Jeong and YB Kim Investigation and Visualization: KH Jeong Supervision: YB Kim and YB Choi Validation: YB Choi Writing-original draft: KH Jeong Writing-review and editing: YB Kim All authors read and approved the final manuscript. Fig. 1.Esophagogastroduodenoscopy finding (day 1) of no evident gastric and duodenal involvement (A). Colonoscopy finding of bleeding with a clot (not shown) from an uncertain focus (B). 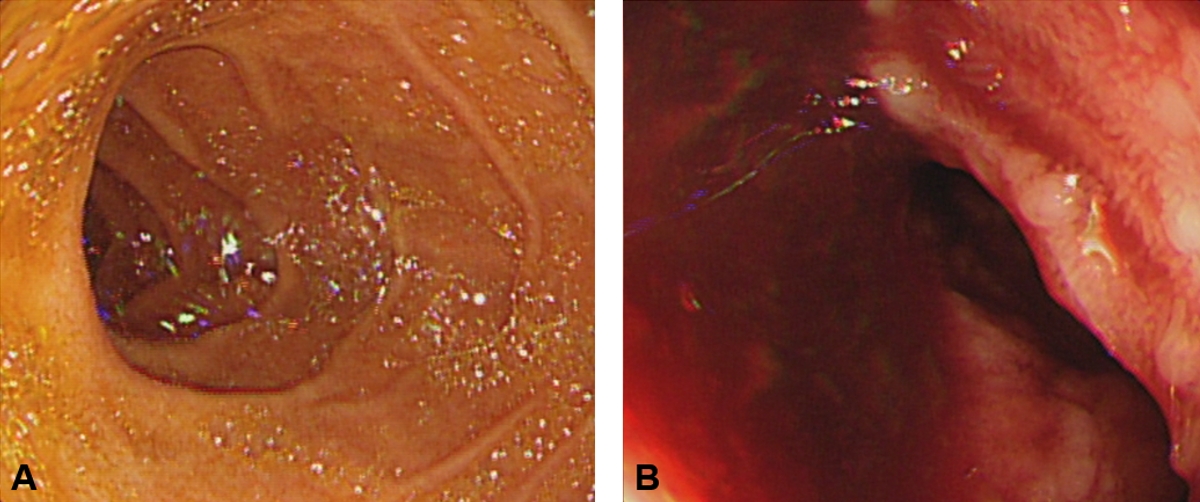
Fig. 2.Capsule endoscopic findings (day 4) showing no evident proximal small bowel involvement (A) and blood-tinged mucosa on the distal ileum (B). 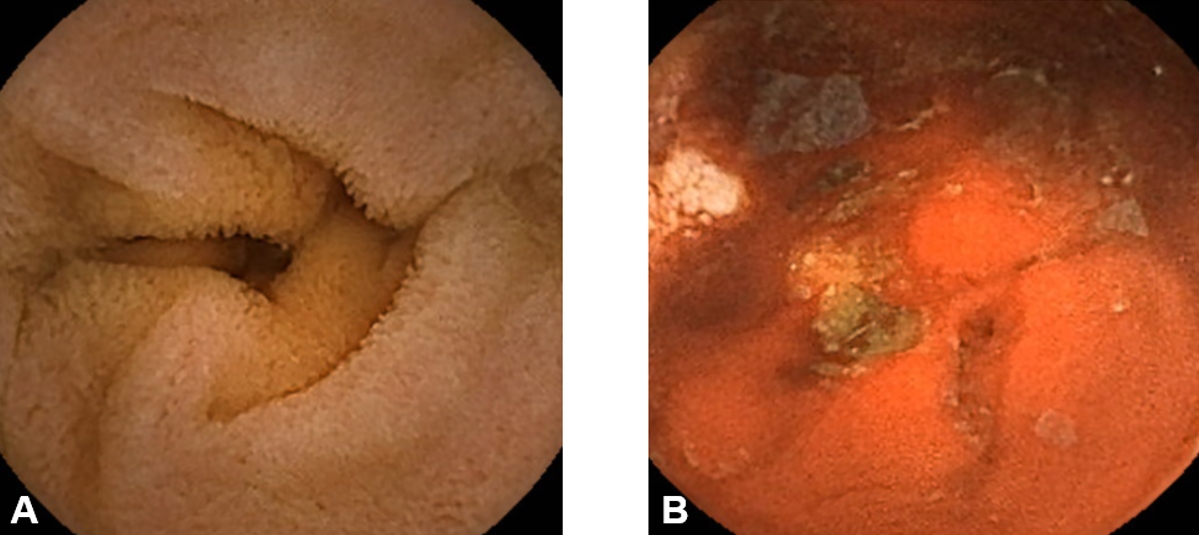
References1. Yoon HS, Han Y, Kim YJ, Kim MJ, Byun JM, Youk T, et al. Epidemiology of congenital bleeding disorders: a nationwide population-based Korean study. J Korean Med Sci 2020;35:e350.
2. Korea Hemophilia Foundation. Disease history: 14. Enrolled patients by disease In: Korea Hemophilia Foundation. 2018 Annual report. Korea Hemophilia Foundation; 2018. p. 14.
3. Wang K, Santiago R. Tranexamic acid - a narrative review for the emergency medicine clinician. Am J Emerg Med 2022;56:33‚Äď44.
4. Lin S, Rockey DC. Obscure gastrointestinal bleeding. Gastroenterol Clin North Am 2005;34:679‚Äď98.
5. Awadie H, Zoabi A, Gralnek IM. Obscure-overt gastrointestinal bleeding: a review. Pol Arch Intern Med 2022;132:16253.
6. Casciani E, Nardo GD, Chin S, Masselli G, Polettini E, Oliva S, et al. MR Enterography in paediatric patients with obscure gastrointestinal bleeding. Eur J Radiol 2017;93:209‚Äď16.
10. Sevenet PO, Kaczor DA, Depasse F. Factor VII Deficiency: From basics to clinical laboratory diagnosis and patient management. Clin Appl Thromb Hemost 2017;23:703‚Äď10.
11. Napolitano M, Siragusa S, Mariani G. Factor VII deficiency: clinical phenotype, genotype and therapy. J Clin Med 2017;6:38.
12. CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23‚Äď32.
13. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105‚Äď16.
14. Schutgens REG, Lisman T. Tranexamic acid is not a universal hemostatic agent. Hemasphere 2021;5:e625.
15. Gluud LL, Klingenberg SL, Langholz E. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev 2012;1:CD006640.
16. Burke E, Harkins P, Ahmed I. Is there a role for tranexamic acid in upper GI bleeding? A systematic review and meta-analysis. Surg Res Pract 2021;2021:8876991.
17. Eghbali A, Melikof L, Taherahmadi H, Bagheri B. Efficacy of tranexamic acid for the prevention of bleeding in patients with von Willebrand disease and Glanzmann thrombasthenia: a controlled, before and after trial. Haemophilia 2016;22:e423‚Äď6.
18. Lanzkowsky P. 15. Disorders of coagulation. Fish JD, Lipton JM, Philip Lanzkowsy P, editors. Lanzkowsky‚Äôs manual of pediatric hematology and oncology 6th ed. Elsevier: 2016. p. 287‚Äď340.
|
|
|||||||||||||||||||||||||||||||||||

 |
 |